Popular Answers (1) You know that in every dynamic 1st order reaction (a reaction with a fixed rate of promotion) we can say that half life is when N/N0=1/2 t1/2= (ln (1/2))/k = ln2/k t1/2=0 Value of `t_(1//2)` for first order reaction is Step by step solution by experts to help you in doubt clearance & scoring excellent marks in examsA second order reaction follows pseudofirst order reaction, when the concentrations of In exothermic Reaction, if we increase the temperature in the exothermic reaction the rate of reactionwill A zeroorder reaction is a reaction having rate of concentration of reactant

A First Order Reaction Takes 40 Min For 30 Decomposition Calculate Its T1 2 Value Brainly In
T1/2 for first order reaction is
T1/2 for first order reaction is-T1/2 for first order reaction is (a) 06/k (b) 0693/k (c) 06/k (d) 010/kClick here to find Half life period t1/2 for first order reaction is Half life period t1/2 for first order reaction is



Show That In A First Order Reaction Time Required For Completion Of 99 9 Is 10 Times Of Half Life Of The Reaction Quora
a zero order reaction b Pseudo zero order reaction c First order reaction d Second order reaction Answer a 3 Which of the following is the half life of zero order reaction?Consider the parallel first order reactions given above How many hrs is the halflife for the decay of A A B (t1/2 = 4hrs) B (t1/2 = 12 hrs) W Hint, take k=kl k2 Q8 The following results were obtained during kinetic studies of the reaction 2A Products k=693x102 The time1162 K views 58 K people like this
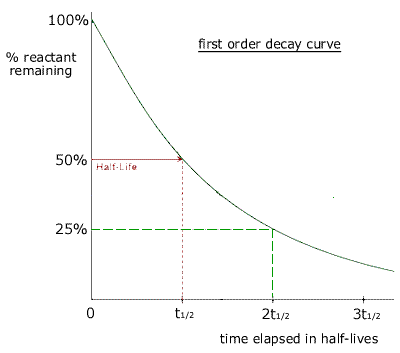
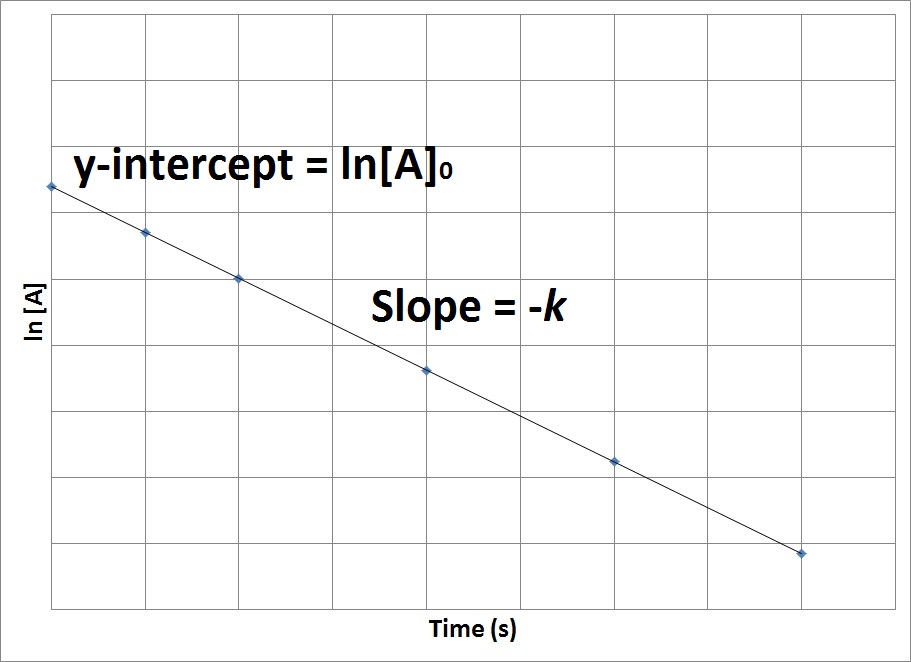
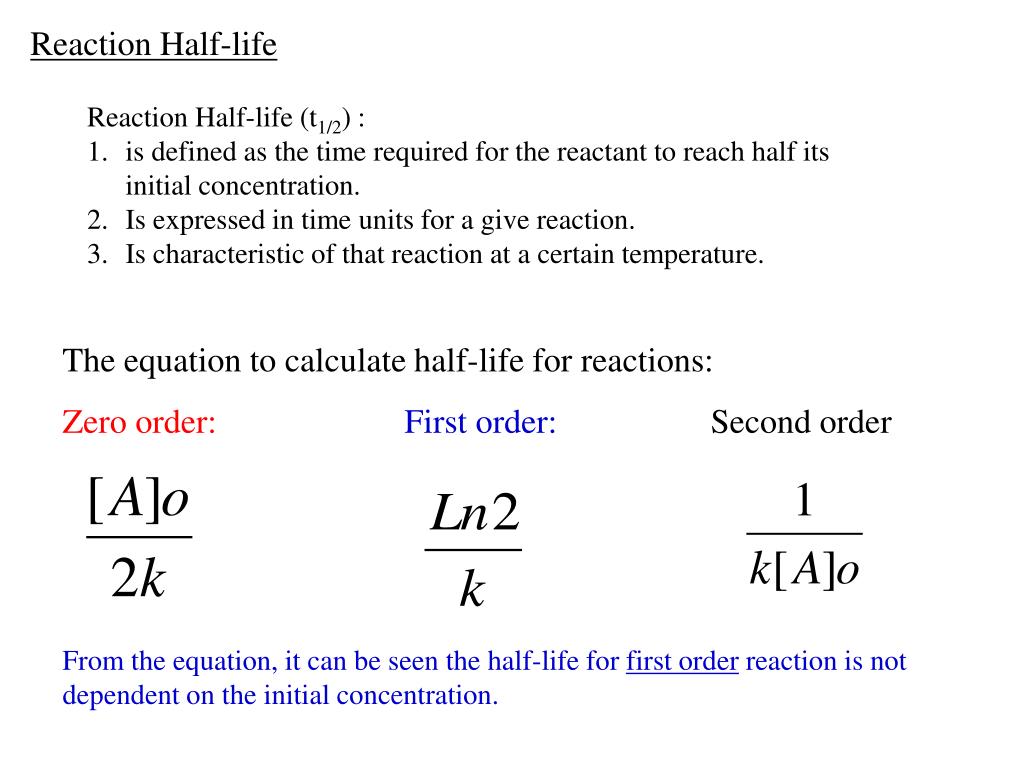
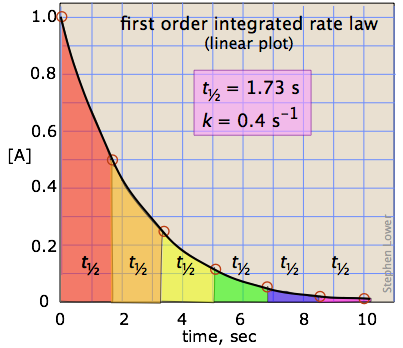
FirstOrder Kinetics In First order reactions, the graph represents the halflife is different from zero order reaction in a way that the slope continually decreases as time progresses until it reaches zeroWe can also easily see that the length of halflife will be constant, independent of concentration For example, it takes the same amount of time for the concentration to(ii) By deriving the equation for t 1/2 of firstorder reaction, prove that it is independent of initial concentration of its reacting species Hint Fora first ortler reaction,A t1/2 = A0 /2k b t1/2 = 0693/2k c t1/2 = A0 /2 d t1/2 = 2k/ A0 Answer a 4 The unit of k for zero order reaction is a moles/litre/second b moles c moles
Sep 29,21 Read the passage given below and answer the following questionsThe halflife of a reaction is the time required for the concentration of reactant to decrease by half, ie,At = A/2For first order reaction,t1/2 = 0693/kThis means t1/2 is independent of initial concentration Figure shows that typical variation of concentration of reactant exhibiting first order kineticsGet answer Value of t_(1,,2) for first order reaction is Apne doubts clear karein ab Whatsapp par bhi Try it nowI know to use the ln(k1/k2)= (ea/R)((1/T1)(1/T2)) but how do i know what T2 is when i only Chem2 A reaction in which A, B, and C react to form products is zero order in A, onehalf order in B, and second



Kinetics 6 34 Half Life




The T1 2 Of A First Order Reaction Is 60 Minutes What Percentage Will Be Left After 240 Minutes
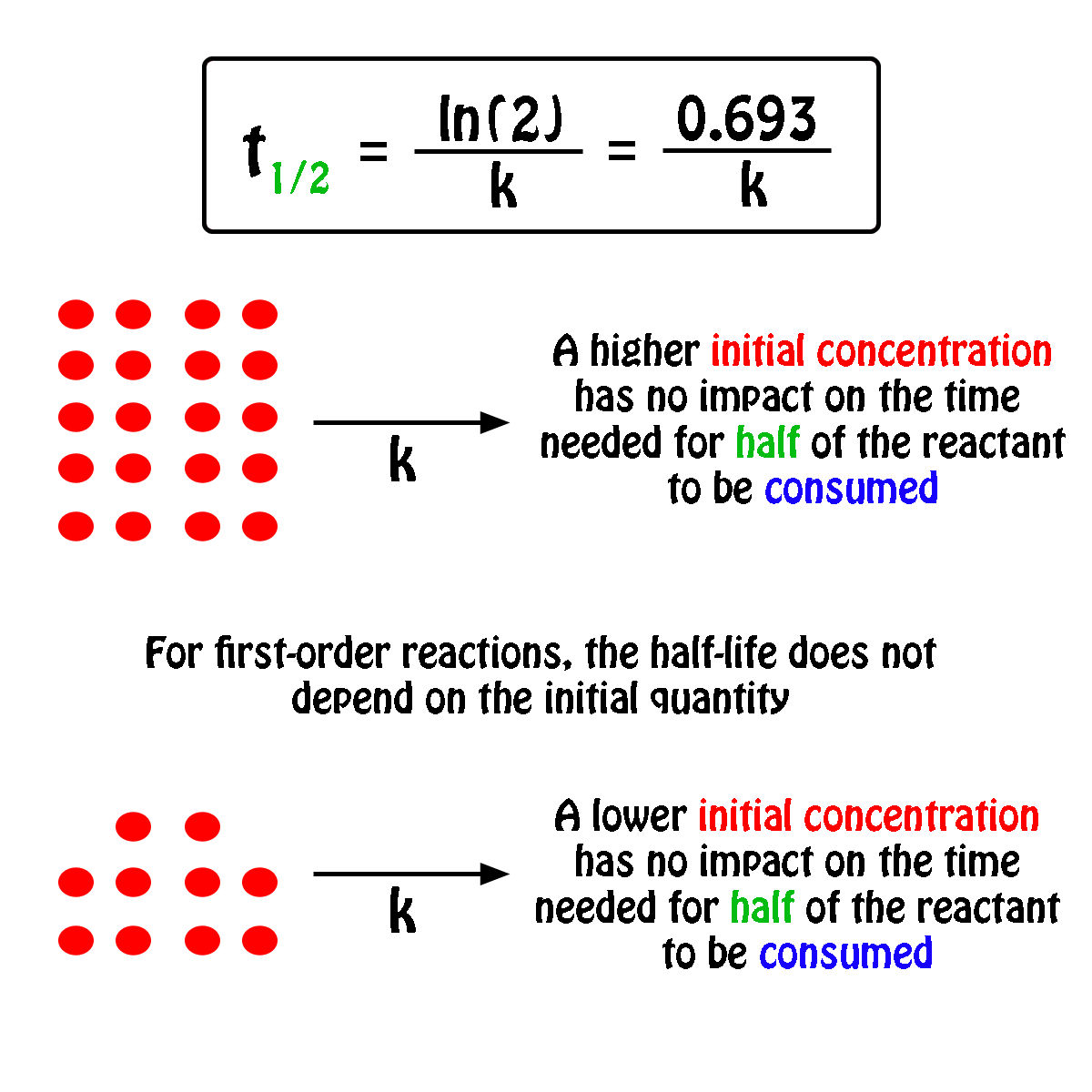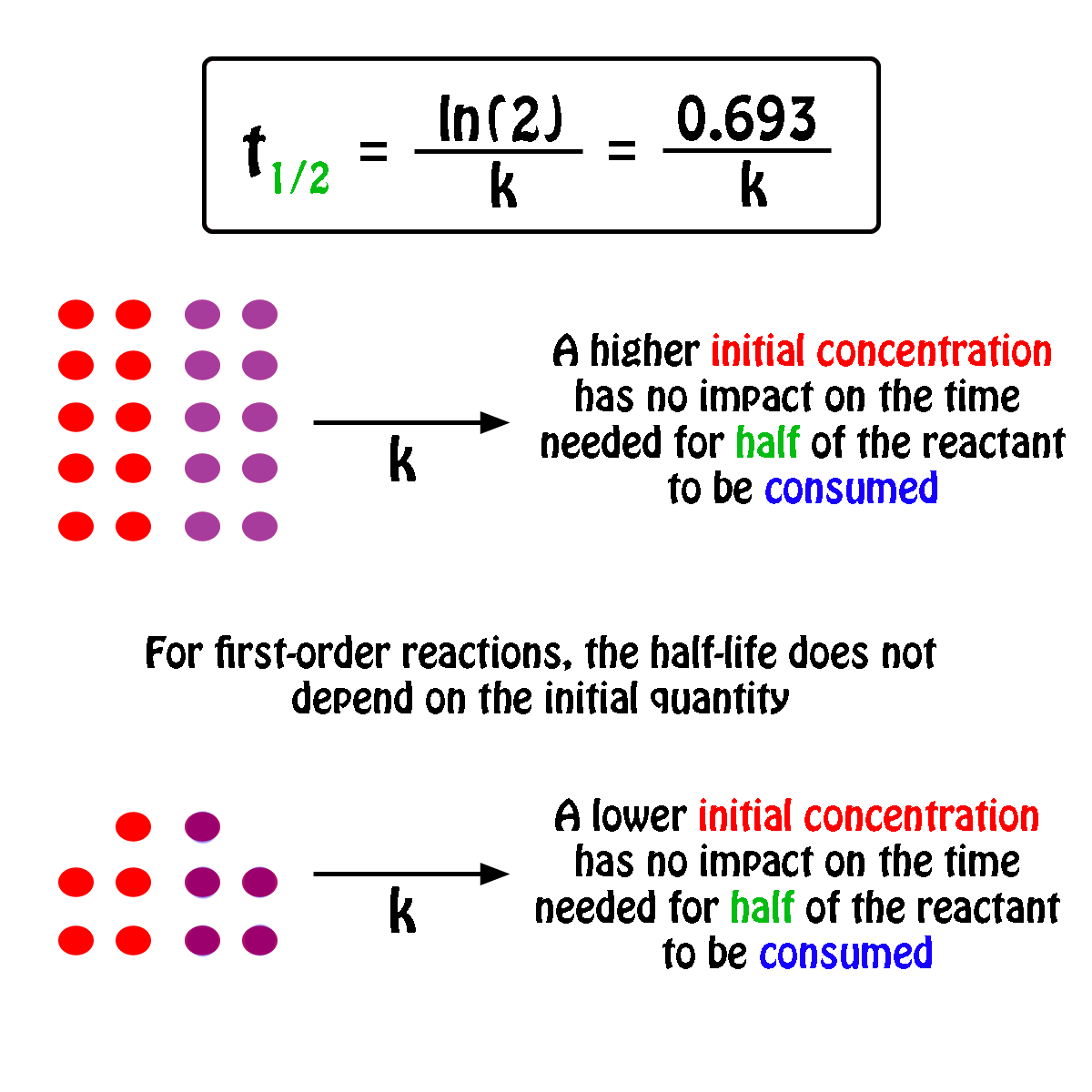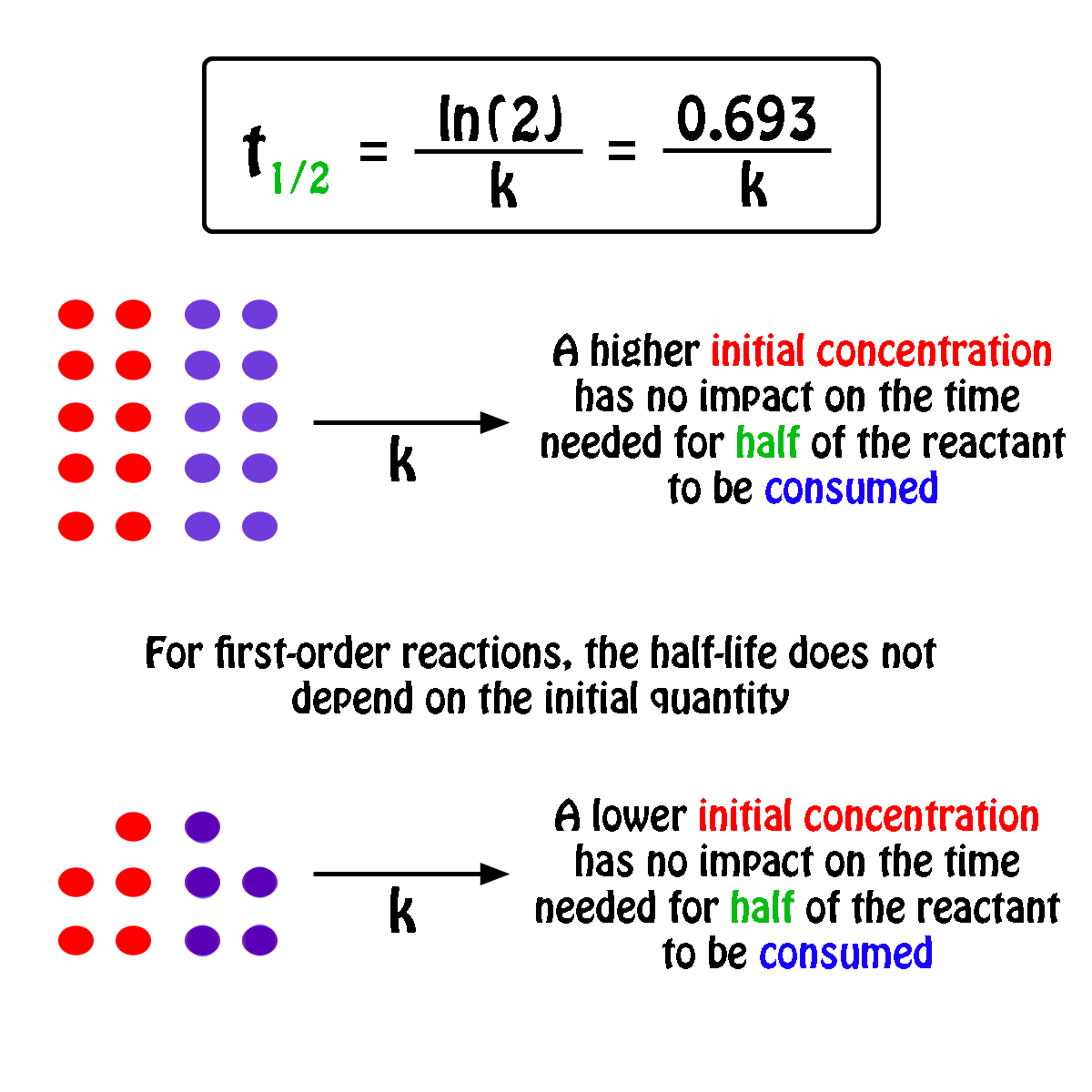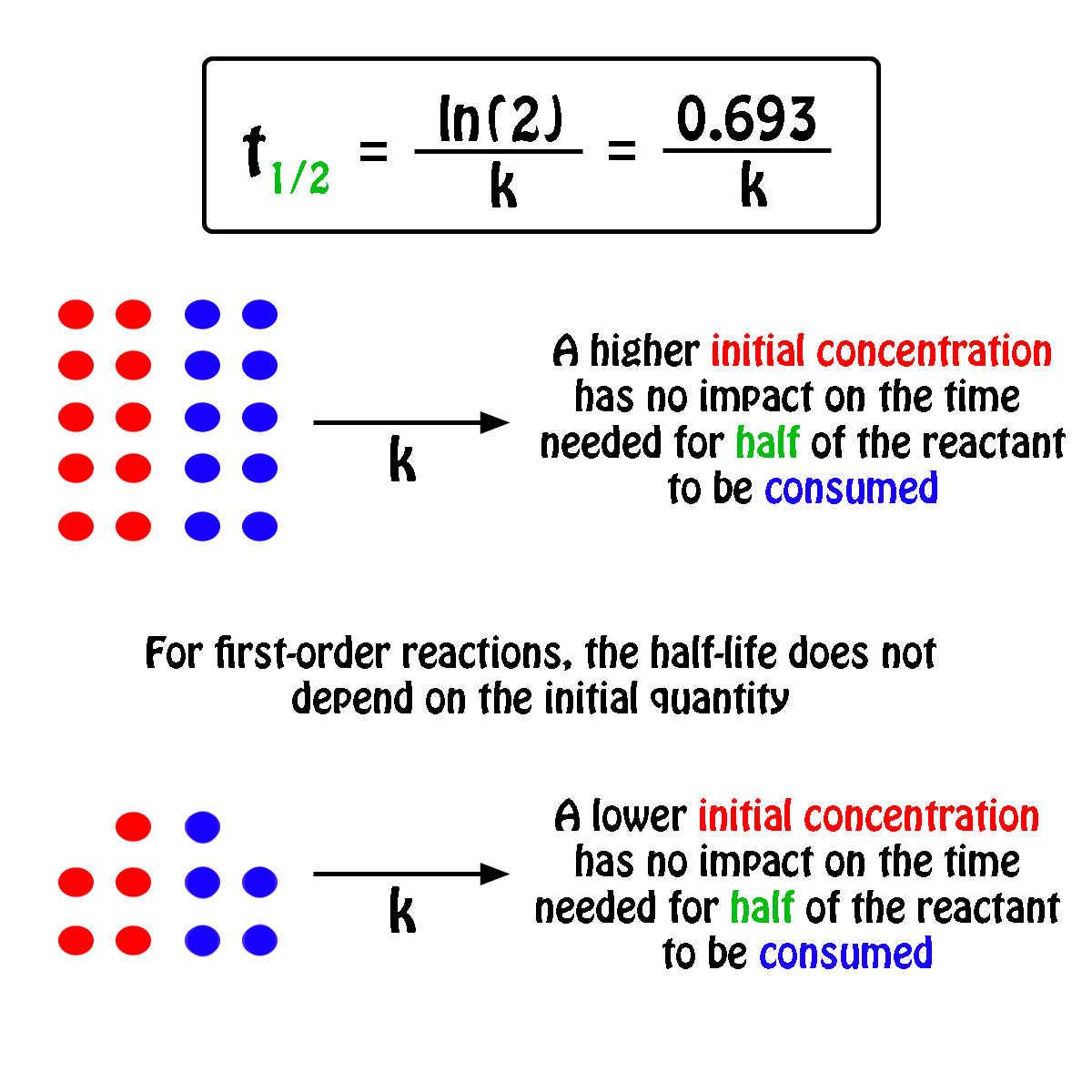
The halflife of a firstorder reaction is a constant that is related to the rate constant for the reaction t 1/2 = 0693/ k Radioactive decay reactions are firstorder reactions The rate of decay, or activity, of a sample of a radioactive substance is the decrease in the number of radioactive nuclei per unit time For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction Q Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 10 g of polymer ofFor a first order reaction, the ratio of t_(1//2) to t_(3//4) is Apne doubts clear karein ab Whatsapp par bhi Try it now CLICK HERE 1x 15x 2x Loading DoubtNut Solution for you Watch 1000 concepts & tricky questions explained!




First Order Reaction An Overview Sciencedirect Topics



Concentration Time Relationships Integrated Rate Laws Introductory Chemistry
Question Halflife Equation For Firstorder Reactions T1/2=0693k Where T1/2 Is The Halflife In Seconds (s), And K Is The Rate Constant In Inverse Seconds (s−1) A What Is The Halflife Of A Firstorder Reaction With A Rate Constant Of 290×10−4 S−1? The differential equation describing firstorder kinetics is given below (231) R a t e = − d A d t = k A 1 = k A The "rate" is the reaction rate (in units of molar/time) and k is the reaction rate coefficient (in units of 1/time) However, the units of k vary for nonfirstorder reactions These differential equations areHalf life of first order reaction is constant 100 to 50 will take 1 half life 50 to 25 will take 1 half life Therefore, 2 half lives (4 minutes) will be taken for a first order reaction to



Cbse Class 12 Chemistry Notes Chemical Kinetics Aglasem Schools



Half Lives
Get answer Value of `t_(1//2)` for first order reaction is This browser does not support the video elementAnswer For 1st order reaction, rate constant, k = (2303/t)×log(Ao/A) = (2303/40)×log{Ao/(Ao025Ao)} = 0055×log(4/3) = 7278 × 10^3 /min Now, tGiven Half life (T1/2) = 10 min For a first order reaction, the relation between half life ( T1/2) and rate constant k is Thus, k = 0693/ T1/2 = 0693/10 = min^1 Consider the initial concentration to be 100%, hence after 80% completion



1




A First Order Reaction Is Half Complete In 45 Min Caluclate The Rate Constant For The Reaction Find 6 Answers Solutions Learnpick Resources
Find an answer to your question A first order reaction takes 10 min for 25% decomposition calculate t1/2 for the reactionGet answer For a first order reaction R rarr P t_(1,,2) is proportional to Of 50% of reaction gets completed in 16 minutes what fraction of reaction would occur in 32 minutes Please solve question 25 Write integrated rate expression for the first order reaction Also, findHalf life for this expression the given questions rate equation



What Is The Ratio Of T3 4 T1 2 For A First Order Reaction Chemistry Topperlearning Com Qfujetmm



2
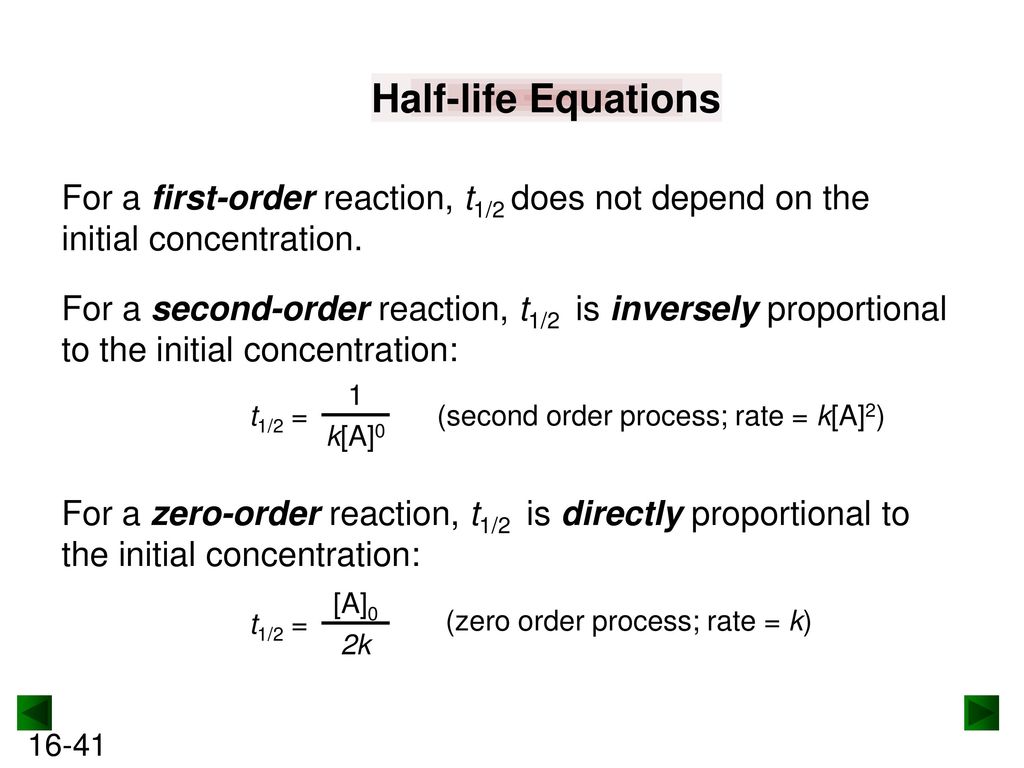
T1/2=0693/k For a secondorder reaction, the halflife depends on the rate constant and the concentration of the reactant and so is expressed as t1/2= 1/kA 1 A certain firstorder reaction (A> Productus) has a rate constant of 7×103 s^1 at 45 degrees celsius A certain firstorder reaction has a rate constant of 275 102 s−1 at °C What is the value of k at 50°C if Ea = 755 kJ/mol?2 t 1 / 2 for a first order reaction is 1426 m i n Calculate the time when 5 % of the reactant is left UP CPMT 15 3 The inversion of cane sugar is first order in sugar and proceeds with halflife of 600 minutes at pH = 4 for a given concentration of sugar




A First Order Reaction Takes 40 Min For 30 Decomposition Calculate T1 2 Half Life Time Doctor Logics




3 Correct Statement About First Order Re Physical Chemistry
For a firstorder reaction, the halflife period (t 1/2) is independent of initial concentration of its reacting species (i) What is meant by halflife period of a reaction?Manipal 09 t1/2 for a first order reaction is 10 min Starting with 10 M, the rate after min is (A) text M text min1 (B) × 5For first order reaction, we know that at half life of reaction, t = t ½ & A = A o /2 So Since k is a constant for a given reaction at a given temperature and the expression lacks any concentration term so halftime of a 1st order reaction is a constant independent of initial concentration of reactant This means if we start with 4 mole L –1 of a reactant reacting by firstorder




A First Order Reaction Takes 40 Min For 30 Decomposition Calculate T 1 2 Given Youtube



Question 19 The Half Life T1 2 Of A First Order Chegg Com
Click here👆to get an answer to your question ️ t1/2 for a first order reaction is 693 s , the value of rate constant for the reaction would be 10s^1A First Order Reaction Takes 10 Minutes for 25% Decomposition Calculate T1/2 for the Reaction (Given Log 2 = , Log 3 = , Log 4 = ) The t1/2 for a first order rxn is sthe time required for 999 percent decomposition is of the following reaction at 250 °C using the data in this table The standard enthalpy of the reaction, ΔH°rxn, is –442 kJ·mol–1 C2H4(G) The halflife for the firstorder decomposition of is




Find The Ratio T7 8 T1 2 For First Order Reaction Brainly In



A First Order Reaction Is 75 Completed In 40 Minutes Calculate Its T1 2 Sarthaks Econnect Largest Online Education Community
Let ( {t}_{frac{1}{2}}= {t}_{1})Half life period of first order reaction ( k=frac{0693}{{t}_{1}}) ____(i)Let the initial concentration ( = 1)time ( = {t}_{2T1/2=1/kA0 (1/2 &0 are subscript) Consider the general reaction A firstorder reaction has a rate constant of 300 × 103 s1 The time required for the reaction to be 750% complete is 462 s Cyclopropane is converted to propene in a firstorder processHalf life equation for first order reactions t1/2 = 0693/kwhere t1/2 is the half life in second and k is the rate constant ininverse seconds A what is the half life of a first order reaction with a rateconstant of 510 * 10^4/s




First Order Tnt Degradation Rate Constant K H A1 And Half Life Download Table



For A Certain First Order Reaction T1 2 100 Sec How Long Will It Take For The Reaction To Complete 75 Quora
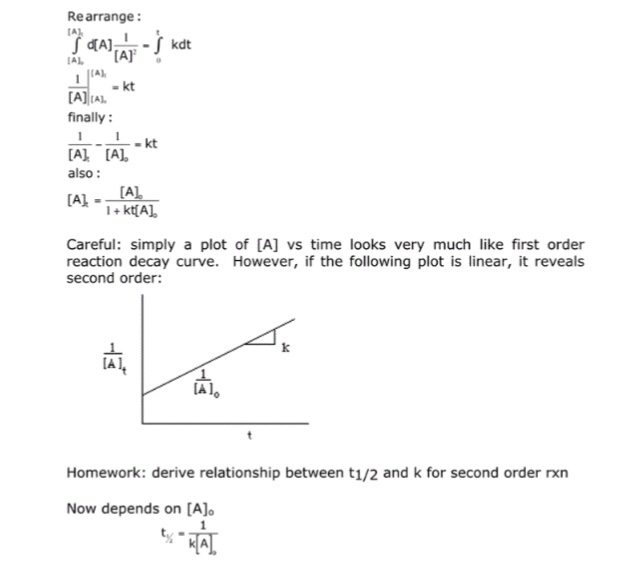
Jack, Jill, and you are in a physical chemistry class The professor writes the following equations on the black board A → B A = A0e − kt The professor assigns you to derive the firstorder reaction in front of the class Jack was assigned to find the rate constant if the reaction halflife is 10 hours The ratio of for first order reaction is 3 1 Explanation Expression for rate law for first order kinetics is given by where, k = rate constant t = age of sample a = let initial amount of the reactant a x = amount left after decay process a) of reaction b) of reaction Learn more about first order kineticsHalf Life (t1/2) Half Life of a reaction is time required to reduce the initial concentration to half The halflife of a reaction depends on the order of reaction The variation of halflife with order is given as t1/2 ∝ A01n Where, A0 = initial concentration n = Order of reaction



A First Order Reaction Takes 40 Min For 30 Decomposition Calculate T1 2 Sarthaks Econnect Largest Online Education Community
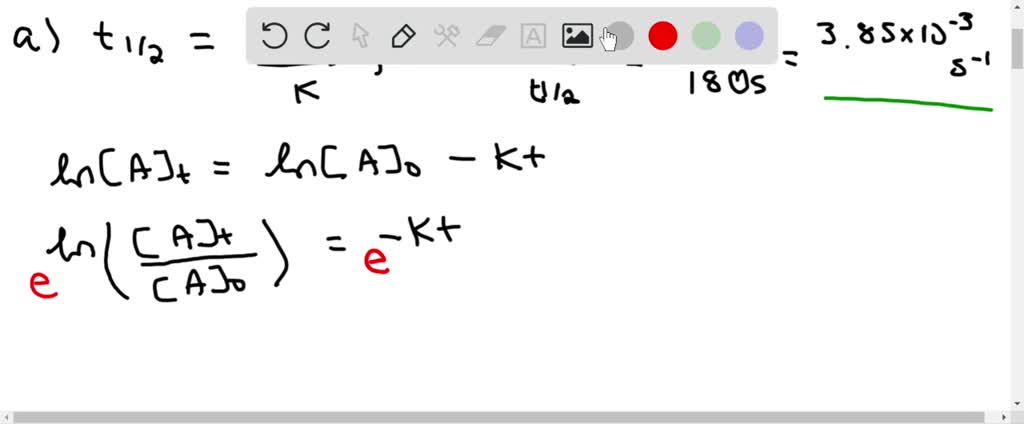



Solved The First Order Reaction A Longrightarrow Products Has T 1 2 180 Mathrm S A What Percent Of A Sample Of A Remains Unreacted 900 S After A Reaction Has Been Started B What Is
Halflife equation for firstorder reactions t1/2=0693k t1/2=0693k where t1/2t1/2 is the halflife in seconds (s)(s), and kk is the rate constant in inverse seconds (s−1)(s−1) Part A What is the halflife of a firstorder reaction with a rate constant of 440×10−4 s−1 s−1?Express your answer with the appropriate units t_color(navy)(1/2) = (ln(2)) / (2 ln(2) ln(3)) * t_(color(navy)(3/4)) ~~ 241 * t_(color(navy)(3/4)) The expression "N" (t) = "N"_0 * e^(t/lambda) gives the quantity of the reactant remaining at time t into a firstorder reaction (The activity regarding the number of reactant particles converted per unit time is directly related to its concentration in the mixture, or




Using The First Order Integrated Rate Law And Half Life Equations Worked Example Video Khan Academy




In A First Order Reaction What Is The Retio Of T 15 16 T 1 2 Brainly In
2 t 1 / 2 for a first order reaction is 1426 m i n Calculate the time when 5 % of the reactant is left 3 The inversion of cane sugar is first order in sugar and proceeds with halflife of 600 minutes at pH = 4 for a given concentration of sugar However, if pH = 5, the halflife changes to 60 minutesB What Is The Rate Constant Of A Firstorder Reaction That Takes 241 Seconds For The Reactant Concentration 2 Determine the order of the reaction and the reaction constant, k, for the reaction using the tactics described in the previous problem The order of the reaction is second, and the value of k is M2 s1 Since the reaction order is second, the formula for t1/2 = k1A o1 This means that the half life of the reaction is



2



Ppt Chapter 16 Powerpoint Presentation Free Download Id
T1/2 for first order reaction is 25 s,what is time required for 10g of reactant to get reduced to 125g Get the answers you need, now!It can be answered in two ways First , by mathematical solution(equation based) Secondly , by verbal solution(concept based) Mathematical solution is simple just In first order reaction, if α is the degree of dissociation, therefore Plot of reciprocal concentration of the reactant vs time is linear, for completion of 75% of reaction of first order, 2 x t 1/2 time is required, for first order reaction dimensions of preexponential factor 'A' are dimensions of ie, T1



Chapter 16 Kinetics Rates And Mechanisms Of Chemical Reactions Ppt Download



2 3 First Order Reactions Chemistry Libretexts
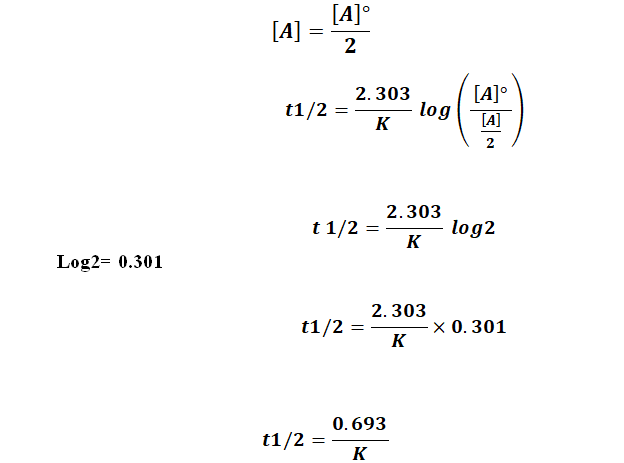



Half Life Formula




A First Order Reaction Takes 40 Minutes For 30 Decomposition Calculate T1 2 For This Reaction Given Log 1 428 0 1548



Solved Derive An Relationship Between T1 2 And K For A Chegg Com




Atomic Structure Notes




First Order Reactions Study Material For Iit Jee Askiitians




T99 9 10 T1 2 In A First Order Reaction Youtube




Chapter 12 Chemical Kinetics Ppt Download




For A First Order Reaction Time Taken For Half Of The Reaction To Complete Is T 1 And 3 4 Of The Reaction To Complete Is T 2 How Are T 1 And T 2 Related




Half Life Equation For First Order Reactions T1 2 0 693 K Clutch Prep




Solved A First Order Reaction Takes 40 Min For 30




Rate Equation Wikipedia



What Is A First Order Half Life Socratic
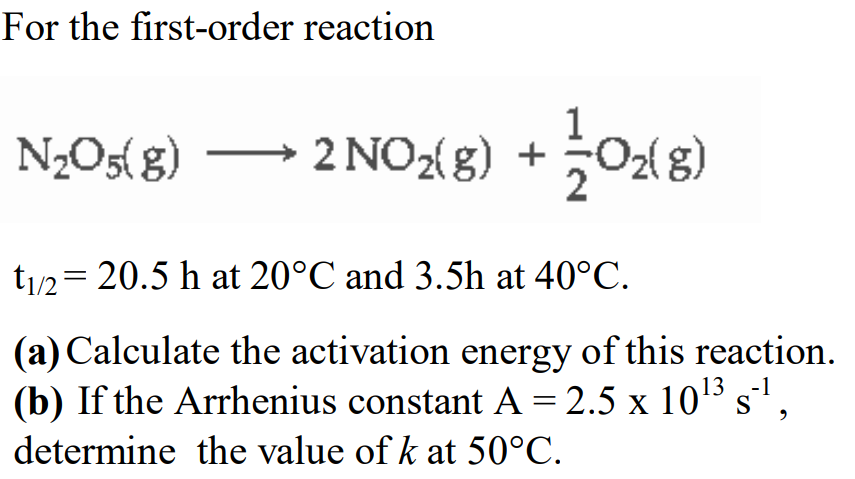



Answered For The First Order Reaction N2o5 G Bartleby
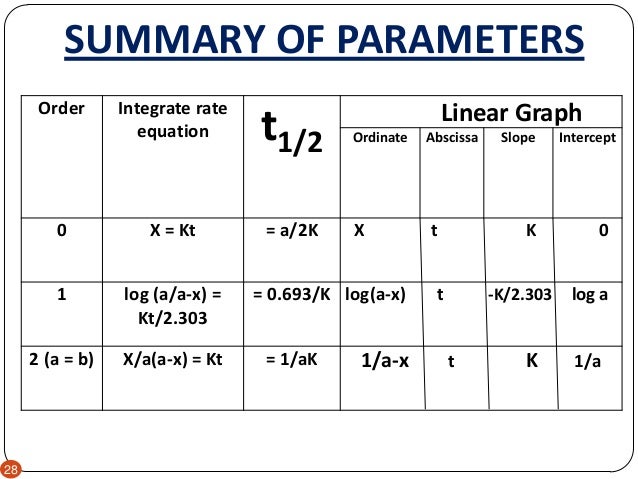



Order Reaction S J Shah




Second Order Reaction Definition And Derivation For Rate Law And Half Life



2




Pseudo First Order Reaction Rate Constants Half Life And Adsorption Download Table



Chm 1046




Half Life Equation For First Order Reactions T1 2 0 693 K Clutch Prep



Ncerthelp Blogspot Com




Iit Jee First Order Reaction In Hindi Offered By Unacademy




Oneclass Part A Half Life Equation For First Order Reactions T1 2 0 693k Where T1 2 Is The Half Lif
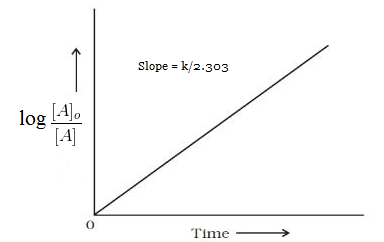



First Order Reactions Study Material For Iit Jee Askiitians




Half Life Introduction To Chemistry
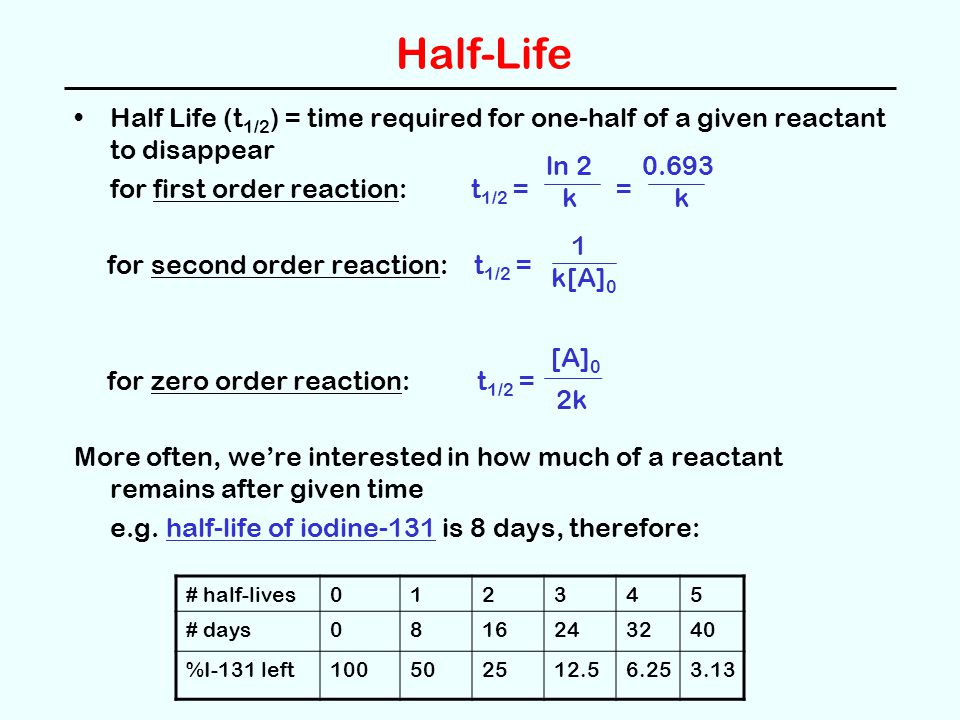



Reaction Rates Chapter 13 Ppt Video Online Download




Half Life Of A First Order Reaction Video Khan Academy




Half Life Equation For First Order Reactions T1 2 0 693 K Clutch Prep
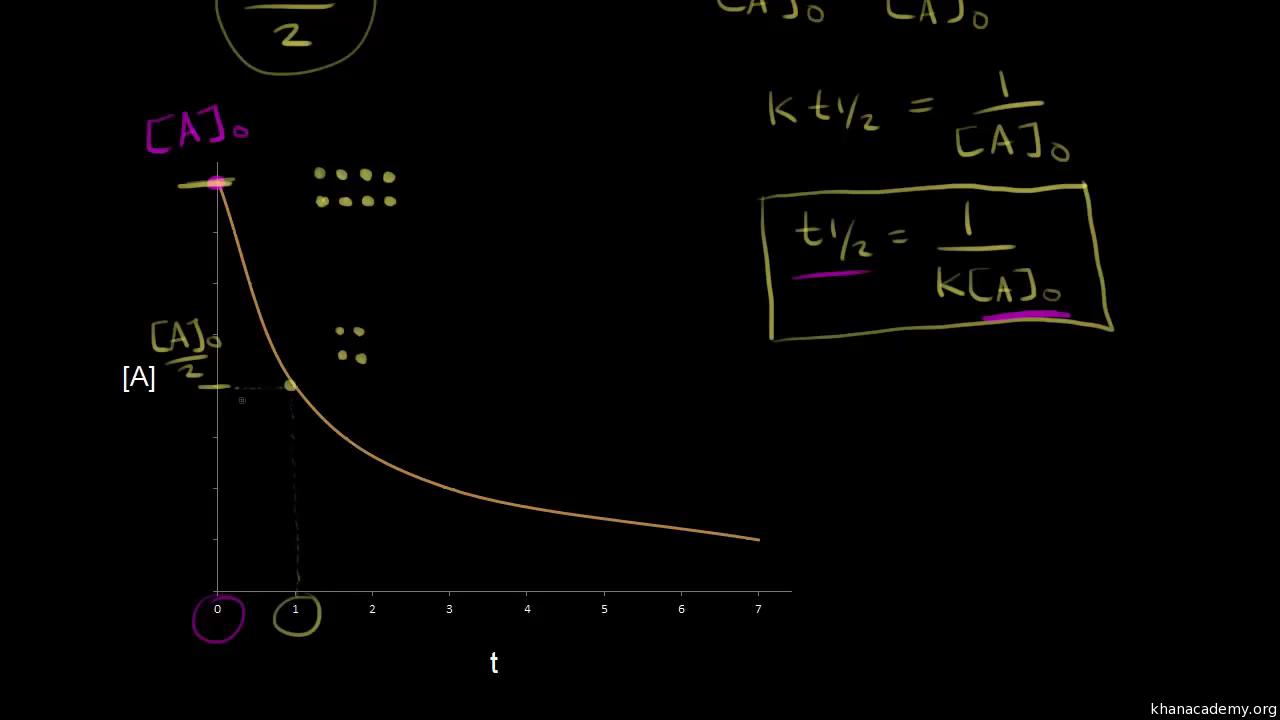



Half Life Of A Second Order Reaction Video Khan Academy



1




A First Order Reaction Takes 10 Minutes For 25 Decomposition Calculate T1 2 For The Reaction Chemistry Chemical Kinetics Meritnation Com




Zero Order Reaction Definition Derivation Graph Examples




Answered For The First Order Reaction N2o5 G 2 Bartleby




The Plot Of T1 2 V S R 0 For A Reaction Is A Straight Line



Show That In A First Order Reaction Time Required For Completion Of 99 9 Is 10 Times Of Half Life Of The Reaction Quora



A First Order Decomposition Reaction Takes 40 Minutes For 30 Decomposition Calculate Its T1 2 Value Chemistry Topperlearning Com Elp9g33gg




Rate Equation Wikipedia




What Is The Ratio Of T3 4 T1 2 For A First Order Reaction Chemistry Topperlearning Com Qfujetmm




For A Reaction A Arrow Products A Plot Of Log T1 2 Versus




Chem Kinetics Online 18 Q 9 For A First Order Reaction A P T12 Half Life Is 10 Days Chemistry Chemical Kinetics Meritnation Com




The Half Life Of A Reaction T1 2 Is The Time It Takes For The Reactant Concentration A Homeworklib



Half Life Of




Solved What Is The Ratio Of T1 2 To T1 3 For A First Order Reaction




For A First Order Reaction The Ratio Of T 1 2 To T 3 4 Is
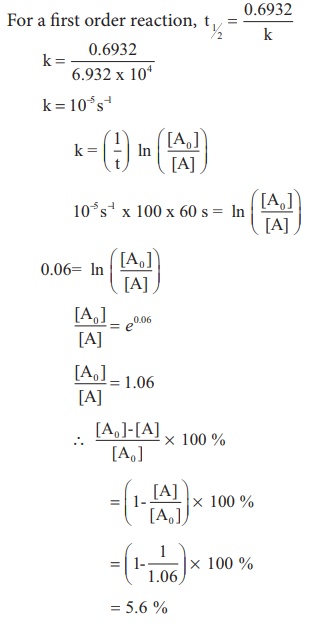



Half Life Period Of A Reaction Chemical Kinetics



What Is The Effect Of Reactant Concentration On The Half Life Of A First Order Reaction Quora



2




The Pseudo First Order Reaction Rate Constant K And T1 2 Download Scientific Diagram




A First Order Reaction Takes 40 Min For 30 Decomposition Calculate Its T1 2 Value Brainly In




The Half Life T1 2 Of A First Order Reaction Is Chegg Com
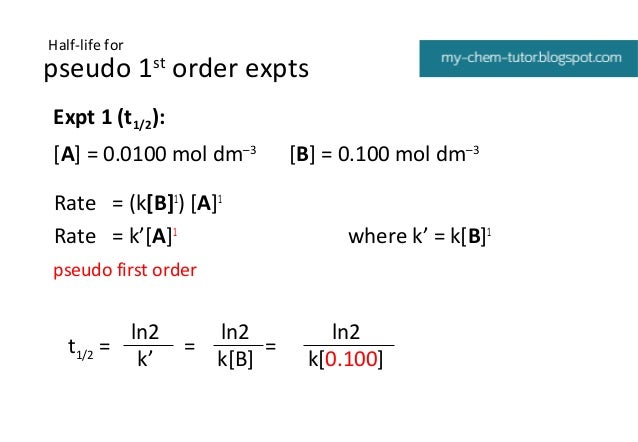



Kinetics Pseudo Order
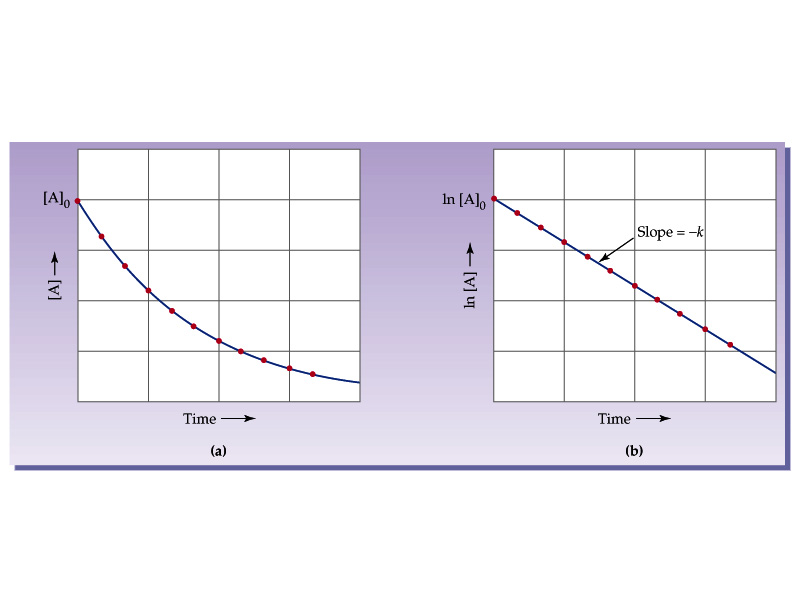



Kinetics




In The First Order Reaction 10 Of The Reactant Is Consumed In 25 Minutes Calculate I The Half Life Period Of The Reaction Ii The Time Required For Completing 87 5 Of The Reaction From Chemistry Chemical




A First Order Reaction Has A Rate Constant Of 1



2
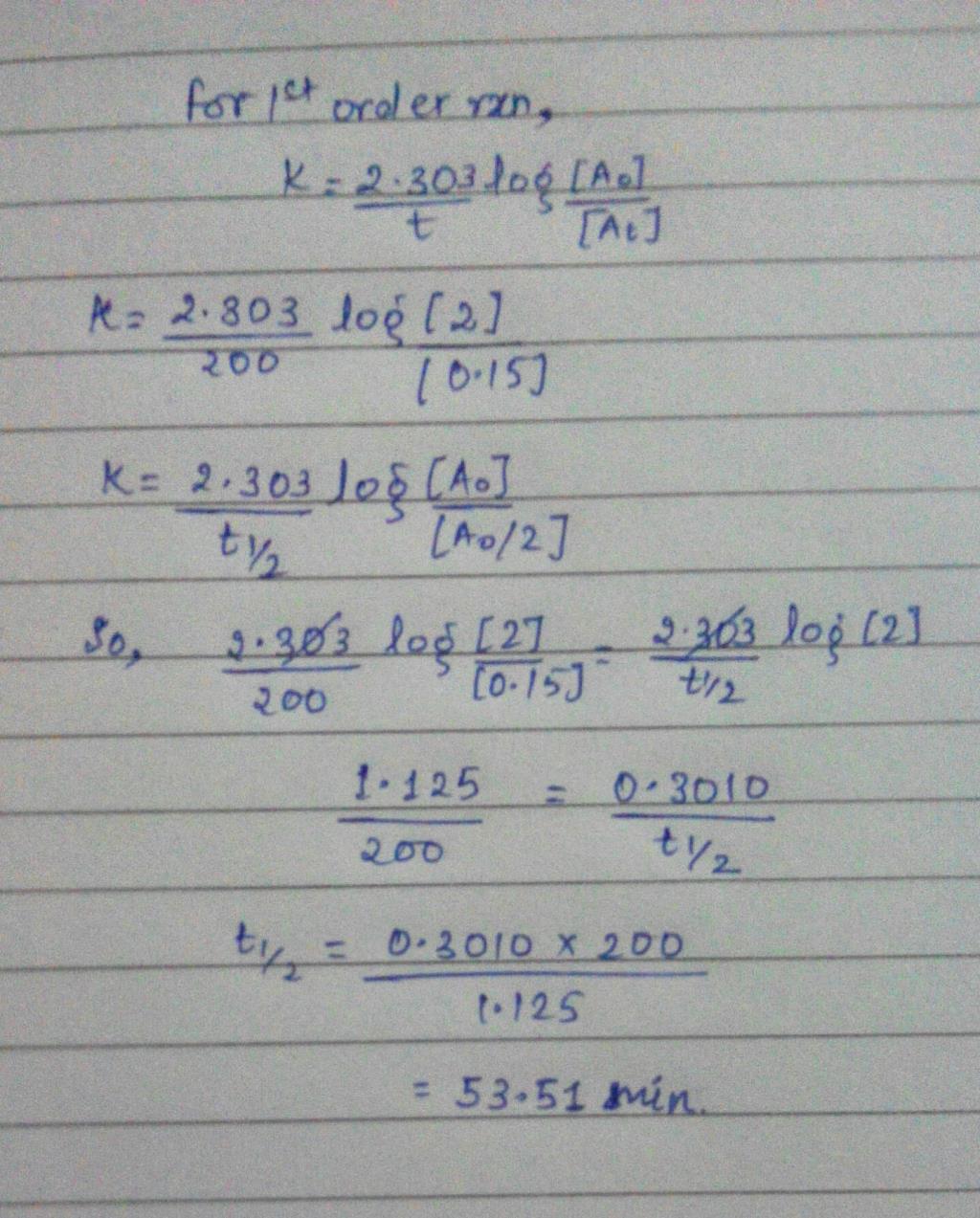



A Substance A Decomposes By A First Or Der Reaction Starting Initially With A 2 00 M And After 0 Min A Becomes 0 15 M For This Reaction T1 2 Is 1995 A 53 72 Minb 50 49



2




Fujn Pages 151 0 Flip Pdf Download Fliphtml5




Pubdoc 11 348




I What Is Meant By Half Life Period Of A Reaction Ii By Deriving The Equation For T1 2 Of First Order Reaction Target Batch




Solved Half Life Equation For First Order Reactions T 2 Chegg Com
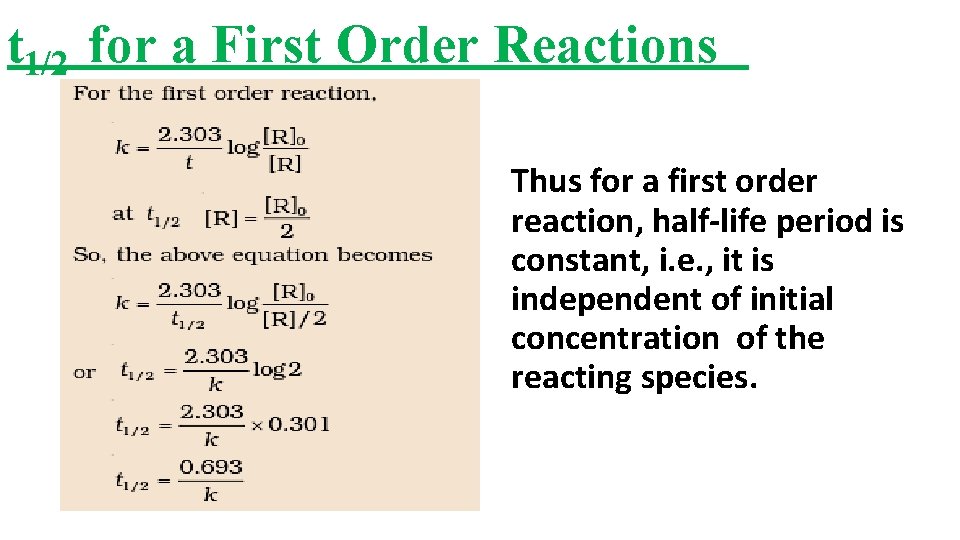



Chemical Kinetics By Jherudden Pgt Chemistry Kv Secl




51 Which Of The Following Represents The Equation For A First Order Half Life A 1 2 Homeworklib




If The T 1 2 For A First Order Reaction 0 4 Min The Time Of Or 99 9 Completion Of The Reaction Is Min




Chemical Kinetics And Stability The Purpose Of Stability
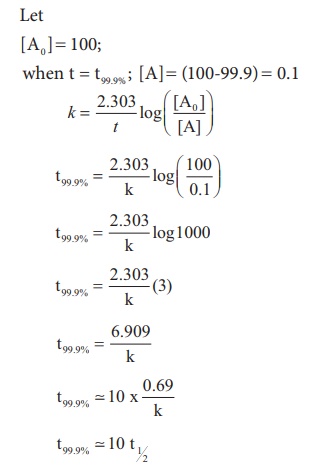



Chemical Kinetics Solved Example Problems Chemistry




In A First Order Reaction 10 Of The Reactant Is Consumed In 25 Minutes Calculate I The Half Life Of The Reaction Ii The Time Required For Completing 17 Of The Reaction




For A First Order Reaction T 7 8 N Xx T 1 2 The Value Of N Will Be



First Order Reaction



Stlawrencehighschool Edu In




First Order Reaction Pdf Pharmacology Drugs




What Is The Ratio Of T3 4 T1 2 For A First Order Reaction Chemistry Chemical Kinetics Meritnation Com




Td 3 Tut Part 3 Droit Constitutionnel General Drpul1000 Ul Studocu




Half Life T1 2 Reference Notes Grade 12 Science Notes
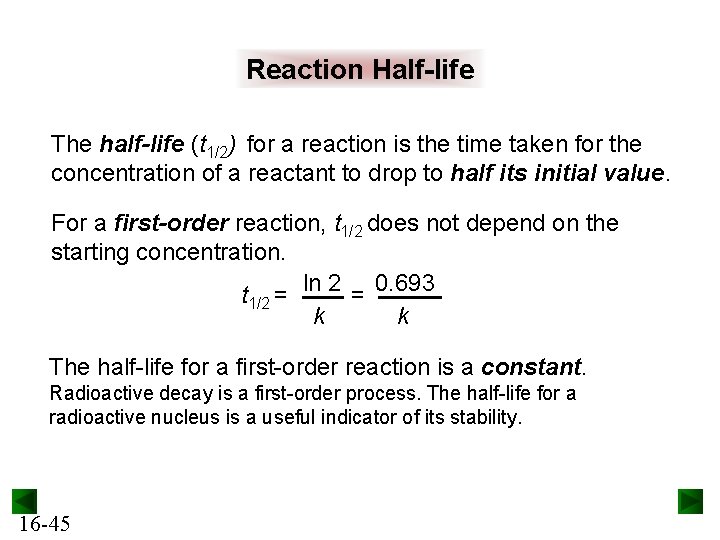



Chapter 16 Kinetics Rates And Mechanisms Of Chemical




Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 0s 1 B 2 Mi N 1 C 4years 1
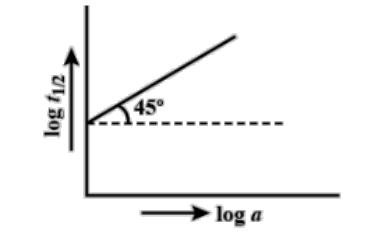



Following Is The Graph Between Log T12 And Log A A Class 11 Chemistry Cbse




Show That For A First Order Reaction T25 0 415 T1 2 Edurev Class 12 Question
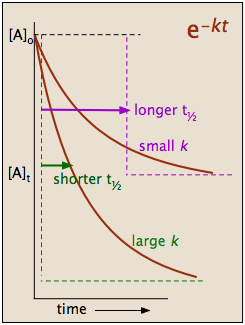



2 3 First Order Reactions Chemistry Libretexts



0 件のコメント:
コメントを投稿